日本語 English
Our Reserch Summary
The molecular mechanism of neurodegenerative diseases such as motor neuron disease (amyotrophic lateral sclerosis, ALS) remains unknown. Therefore, therapeutic strategy has not been established. Our laboratory aims to elucidate the mechanism of onset and progression of motor neuron disease, which have been shown to be derived from the pathological changes within different cell types; motor neurons and glial cells. We will analyze inherited ALS, using mouse, cell culture, and in vitro system as models. Based on these studies, we expect to design the therapeutic interventions for the sporadic ALS patients in future.
We have revealed that non-cell-autonomous effect mediated by glial cells around neurons accelerates disease progression in ALS. Therefore, it is important to understand the role of glial cells to develop a novel therapeutic strategy for ALS. We are also focused on the pathological role of neuroinflammation induced by glial cells in Alzheimer's disease.
FOCUS
Non-cell Autonomous Neurodegeneration
Transgenic mice carrying human SOD1 (superoxide dismutase 1) mutations linked to familial ALS have widely been used for ALS research. In our laboratory, we established LoxSOD1G37R mice, which enable us to eliminate the mutant SOD1 gene from specific cell types, to reveal the cell types involved in ALS onset and progression. Mutant SOD1 in motor neurons determines the ALS onset. On the other hand, mutant SOD1 in glial cells, such as microglia or astrocytes, determines the ALS progression (Boillee et al. Science 2006; Yamanaka et al. Nat Neurosci 2008). These findings indicate that non-cell-autonomous mechanisms induce motor neuron degeneration in ALS (Yamanaka et al. PNAS 2008). We are now striving to elucidate the detailed roles of various cells in the central nervous system on the ALS pathomechanisms.
Our Reserch Projects
A) glial cells, neuroinflammation, systematic homeostasis and neurodegenerative diseases
1. Roles of glial cells, neuroinflammation, gila-immune interaction in ALS and AD
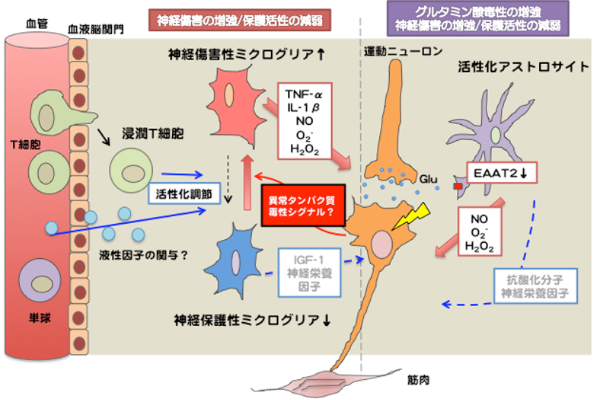
Activated glial cells (astrocytes and microglia) in ALS/AD accelerate neurodegeneration by secreting reactive oxygen species (ROS) or proinflammatory cytokines. Recent studies have revealed that infiltrated immune cells, including T cells, regulate microglial activation to protect neurons. These non-neuronal cells are good targets for a novel therapeutic approach for ALS/AD. Using ALS model mice (mutant SOD1 transgenic mice) and next-generation AD model mice (humanized amyloid precursor protein (APP) with familial AD mutations knocked-in mice), we are developing a method to regulate the non-neuronal cells.
2. Innate or aquired immune systems in ALS and AD pathogenesis
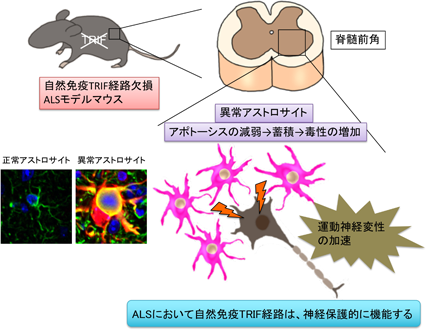
Immune responses can be divided into two major categories: innate immune responses and acquired acquired immune responses involving lymphocytes. Until now, the involvement of innate immune responses in ALS pathogenesis has been unclear. To elucidate the role of innate immune responses, we generated ALS mice lacking MyD88 and TRIF, which are key molecules for the function of Toll-like receptors, sensors of innate immune responses. The results showed that only in the absence of TRIF, the survival time of ALS mice was significantly shortened and aberrant astrocytes accumulated in the lesions. This study revealed a novel function of TRIF in the involvement of the innate immune system in ALS and in the removal of aberrantly activated astrocytes at the lesion (Komine et al. Cell Death Differ 2018). We are currently generating ALS and AD model mice with a systemic immune environment shifted to Th1/2 dominance and analyzing how the systemic immune environment alters glial cells and pathology in the brain.
3. Molecular mechanisms of microglial activation in ALS and AD
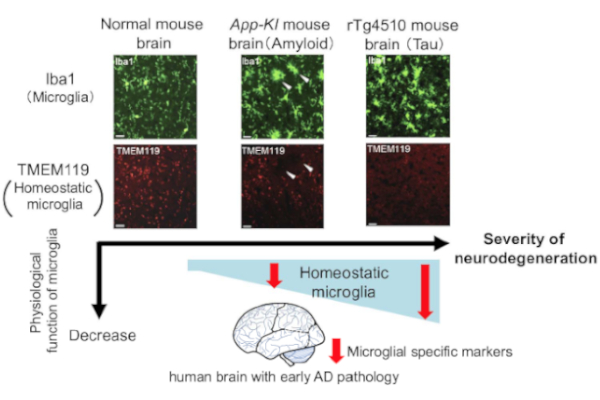
It has been suggested that active microglia, which are innate immune cells in the central nervous system, are devided into tow subtypes: neurotoxic (M1 type) and neuroprotective (M2 type), similar to macrophages. In neurodegenerative diseases such as ALS and AD,switching from M2 to M1 may accelerate disease progression (M1/M2 hypothesis). In addition, the concept of disease-associated microglia (DAM) has recently been proposed. It is remain uncovered whether DAMs are neuroprotective or not. It is also uncertain whether DAMs are general in neurodegenerative diseases, remain unresolved. We are searching for factors that induce active microglial conversion based on DAM and M1/2 hypotheses.
B) Intraneuronal mechanisms of motor neuron degeneration
1. Molecular mechanisms of TDP-43 induced neurodegeneration
TDP-43, an RNA-binding protein, was identified as an abnormally accumulated protein in sporadic ALS (non-hereditary ALS), which accounts for the majority of ALS cases, and frontotemporal lobar degeneration (FTLD), a type of dementia, in 2006. Clarifying the function of TDP-43 and how its abnormalities modulate the motor neurons is the key to understanding the pathogenesis of ALS. We have previously shown that mutant TDP-43 is abnormally stabilized in cells compared to the wild type (Watanabe et al. J Biol Chem 2013). TDP-43 accumulates in Gem, the site of nuclear spliceosome factor maturation, and binds to SMN, the causative gene product of spinal muscular atrophy (SMA), and spliceosome component proteins (snRNPs) aggregate abnormally in sporadic ALS (Tsuiji et al. EMBO Mol Med 2013). We are currently conducting experiments using cultured cells and mice to elucidate the mechanisms that cause these TDP-43 abnormalities.
FOCUS
TDP-43 Monomerization
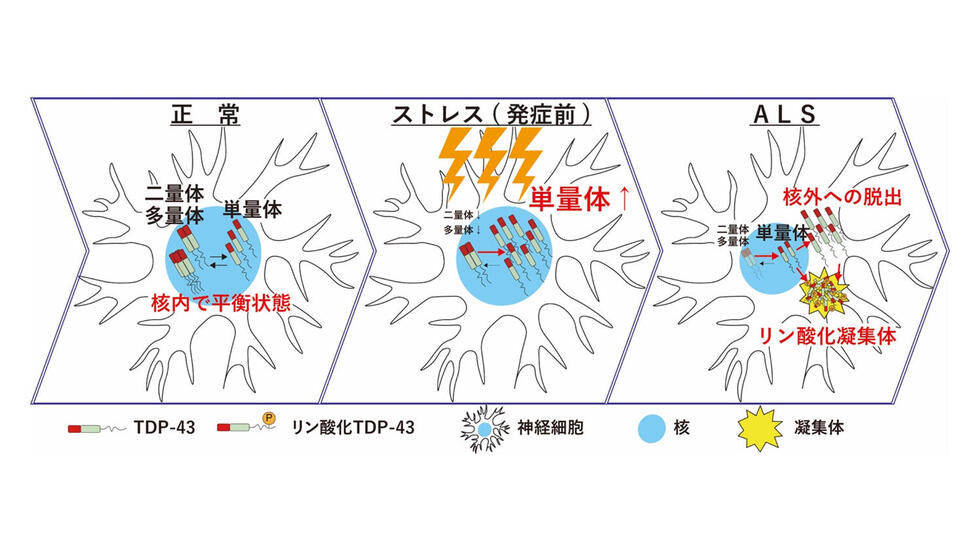
We recently reported that TDP-43 monomerization is a key determinant of TDP-43 pathology in ALS(Oiwa et al. Sci Adv 2023). TDP-43 normally exists in the nucleus as a homomultimer of TDP-43, which plays an important role in the regulation of gene expression. However, when TDP-43 multimerization is disrupted by various stresses on neurons, TDP-43 leaks from the nucleus into the cytoplasm and forms abnormal aggregates, as seen in ALS patients. Inhibition of TDP-43 monomerization could lead to the development of new therapies for ALS, and detection of TDP-43 monomerization could lead to earlier diagnosis and treatment of ALS.
2. disruption of organelle-contacting sites in neurodegenrative diseases
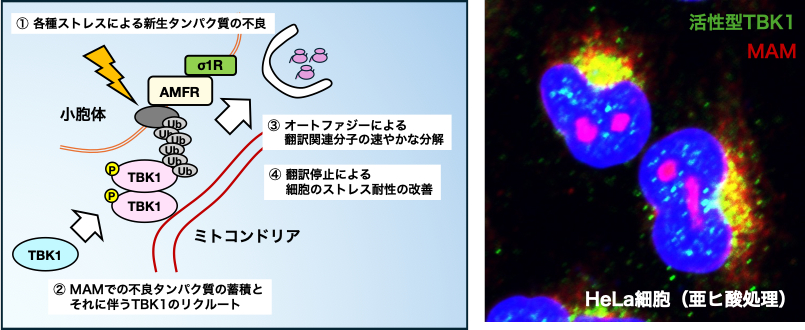
Motor neurons require a large amount of energy to perform their functions due to their long axons and large cell bodies, and mitochondria, organelles involved in energy production, are considered to be important. Recently, knowledge that these organelles interact with each other and that disruption of their interaction (linkage) causes diseases has been accumulating. In our laboratory, we have focused on the disruption of the endoplasmic reticulum-mitochondrial intermembrane region (MAM), the contact between the endoplasmic reticulum (ER), which is important for protein synthesis, and mitochondria, which are important for energy production, and have revealed the importance of MAM disruption in the pathogenesis of ALS. Watanabe et al. EMBO Mol Med 2016, Watanabe & Horiuchi et al. Neurobiol Dis 2023 et al). Disruption of MAM causes neuronal cell death via dysregulation of intracellular Ca2+, and protection of MAM is expected to be a possible treatment for ALS. Recently, we reported that disruption of MAM inhibits the function of TBK1, the ALS-causing gene product, and makes cells vulnerable to the aberrant protein (Watanabe et al. PNAS 2023). Further studies are now underway to elucidate the molecular mechanisms that maintain and disrupt MAM.
We are also conducting research to elucidate the pathophysiology of ALS and AD from various perspectives and to develop therapeutic treatments. If you are interested in our research, please feel free to contact us at the e-mail address below.

